Matrix Landing Mass Spectrometry: Exploring its Applications
Mass spectrometry (MS) is a powerful analytical technique that can identify and quantify molecules based on their mass-to-charge ratio. The traditional approach to MS involves ionizing the sample and analyzing the resulting ions. However, this approach can be limited in terms of sensitivity and selectivity, especially when dealing with complex samples. Matrix Landing Mass Spectrometry (MLMS) is a relatively new technique that overcomes some of these limitations. In this blog post, we will explore the unique points and applications of MLMS.
What is Matrix Landing Mass Spectrometry?
MLMS is a soft ionization technique that involves depositing a matrix onto a sample before ionization. The matrix acts as a mediator to transfer energy to the sample, which results in the generation of ions. The resulting ions are then analyzed using MS. MLMS is a type of Desorption Electrospray Ionization (DESI) technique, which allows for the direct analysis of complex samples without the need for extensive sample preparation.
MLMS involves the deposition of a matrix onto a sample surface using a micro-spotter. The matrix is typically a small molecule that can absorb energy from a laser or other energy source. When the energy is absorbed, the matrix transfers the energy to the sample, which results in the generation of ions. The resulting ions are then analyzed using MS.
The use of a matrix allows for the generation of ions from larger molecules, such as proteins, peptides, and lipids. The matrix also provides a gentle ionization process that reduces fragmentation, which can be a problem with traditional MS techniques. The reduced fragmentation can enhance the sensitivity and selectivity of the analysis by reducing background interference.
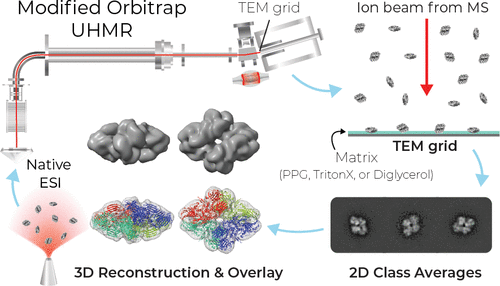
A study from Analytical Chemistry comparing glycerol and poly(propylene) glycol (PPG) matrices for landing showed that PPG had superior performance. Using PPG matrix landing, 3D reconstructions of three protein-protein complexes were obtained.
By Anal. Chem. 2022, 94, 50, 17616–17624 Publication Date: December 7, 2022, https://doi.org/10.1021/acs.analchem.2c04263
Applications of Matrix Landing Mass Spectrometry
Analysis of Complex Samples
One of the unique points of MLMS is its ability to analyze complex samples. Traditional MS techniques often require extensive sample preparation, which can be time-consuming and can lead to sample degradation. MLMS allows for the direct analysis of complex samples, such as biological tissues, without the need for extensive sample preparation. This can save time and reduce the risk of sample degradation.
Detection of Low-Abundance Compounds
MLMS has been shown to be effective in the detection of low-abundance compounds. The use of a matrix can enhance the sensitivity of the analysis by reducing background interference. Additionally, the gentle ionization process can reduce sample degradation and fragmentation, which can lead to the detection of low-abundance compounds that may be missed using traditional MS techniques.
Applications in Drug Discovery and Development
MLMS has several potential applications in drug discovery and development. One such application is the analysis of drug metabolites, which can provide information about the metabolism and clearance of a drug. This information can be used to optimize drug dosing and reduce the risk of adverse effects. MLMS can also be used to analyze drug-protein interactions, which can provide insights into the mechanism of action of a drug.
Another potential application of MLMS in drug discovery and development is the analysis of impurities in drug formulations. Impurities can arise from the manufacturing process or from degradation of the drug over time. MLMS can be used to detect and quantify impurities, which can help ensure the safety and efficacy of a drug.
Applications in Environmental Monitoring
MLMS has potential applications in environmental monitoring. For example, it can be used to detect and quantify pollutants in water or soil samples. MLMS can also be used to analyze the composition of atmospheric particles, which can provide information about sources of pollution and their impact on human health.
Advancements in Matrix Landing Mass Spectrometry
Matrix Landing Mass Spectrometry is a rapidly evolving technique with continued advancements in its use, including:
MALDI-TOF
MALDI-TOF is a variation of MLMS that has been used to analyze proteins in complex samples. This technique involves the use of a laser to ionize the matrix before the ions are analyzed using time-of-flight MS. MALDI-TOF has been shown to be effective in the analysis of proteins in biological tissues.
Tandem Mass Spectrometry
Tandem mass spectrometry (MS/MS) is a powerful analytical technique that involves the use of two mass spectrometers. MLMS has been coupled with MS/MS to enhance the sensitivity and selectivity of the analysis. The use of MS/MS can also provide information about the structure of the analyzed molecules.
High-Resolution Mass Spectrometry
High-resolution mass spectrometry (HRMS) is a technique that allows for the accurate determination of the mass-to-charge ratio of ions. MLMS has been coupled with HRMS to enhance the accuracy of the analysis. The use of HRMS can also provide information about the structure of the analyzed molecules.
Conclusion
Matrix Landing Mass Spectrometry is a relatively new technique that overcomes some of the limitations of traditional MS techniques. It allows for the direct analysis of complex samples without the need for extensive sample preparation. MLMS is particularly useful for the analysis of proteins and peptides, as well as the detection of low-abundance compounds. Additionally, MLMS can be used for imaging mass spectrometry, which allows for the visualization of the spatial distribution of molecules in a sample. As MLMS continues to develop, it is likely that it will find further applications in a range of fields, including drug discovery and development and environmental monitoring.
References:
Römpp A, Karst U. Current trends in mass spectrometry imaging. Anal Bioanal Chem. 2015 Mar;407(8):2023-5. doi: 10.1007/s00216-015-8479-7. Epub 2015 Feb 4. PMID: 25650000.
Nordhoff E, Ingendoh A, Cramer R, Overberg A, Stahl B, Karas M, Hillenkamp F, Crain PF. Matrix-assisted laser desorption/ionization mass spectrometry of nucleic acids with wavelengths in the ultraviolet and infrared. Rapid Commun Mass Spectrom. 1992 Dec;6(12):771-6. doi: 10.1002/rcm.1290061212. PMID: 1283705.